|
Bessemer식 전로(Bessemer式 轉爐; Bessemer converter)
Bessemer process was the first inexpensive industrial process for the mass-production of steel from
molten pig iron. The process is named after its inventor, Henry Bessemer, who took out a patent on
the process in 1855. The process was independently discovered in 1851 by William Kelly.
The process had also been used outside of Europe for hundreds of years, but not on an industrial scale.
The key principle is removal of impurities from the iron by oxidation through air being blown through
the molten iron. The oxidation also raises the temperature of the iron mass and keeps it molten.
|
The details of the process
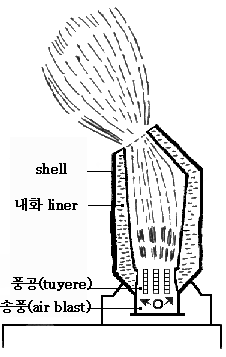
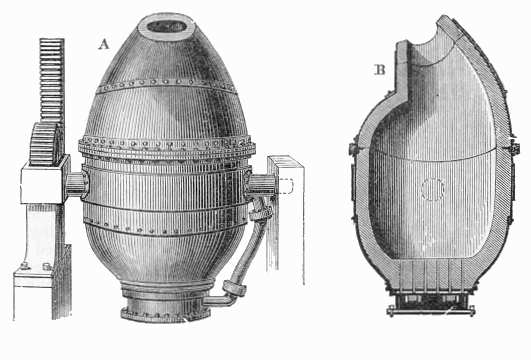
- Bessemer Converter
The process is carried on in a large ovoid steel container lined with clay or dolomite called
the Bessemer converter. The capacity of a converter was from 8 to 30 tons of molten iron with
a usual charge being around 15 tons. At the top of the converter is an opening, usually tilted
to the side relative to the body of the vessel, through which the iron is introduced and the
finished product removed. The bottom is perforated with a number of channels called tuyeres
through which air is forced into the converter. The converter is pivoted on trunnions so that
it can be rotated to receive the charge, turned upright during conversion, and then rotated again
for pouring out the molten steel at the end.
- Oxidation
The oxidation process removes impurities such as silicon, manganese, and carbon as oxides.
These oxides either escape as gas or form a solid slag. The refractory lining of the converter
also plays a role in the conversion - the clay lining is used in the acid Bessemer, in which
there is low phosphorus in the raw material. Dolomite is used when the phosphorus content
is high in the basic Bessemer (limestone or magnesite linings are also sometimes used instead
of dolomite) - this is also known as a Gilchrist-Thomas converter. In order to give the steel
the desired properties, other substances could be added to the molten steel when conversion
was complete, such as spiegeleisen (an iron-carbon-manganese alloy).
- Managing the process
When the required steel had been formed, it was poured out into ladles and then transferred into
moulds and the lighter slag is left behind. The conversion process (called the "blow") was
completed in around twenty minutes. During this period the progress of the oxidation of the
impurities was judged by the appearance of the flame issuing from the mouth of the converter:
the modern use of photoelectric methods of recording the characteristics of the flame has greatly
aided the blower in controlling the final quality of the product. After the blow, the liquid metal
was recarburized to the desired point and other alloying materials are added, depending on the desired
product.
|